
Chemistry, 10.11.2020 09:20, Josephjenkins620
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calculate the mass of 3.33 moles of air.
First, complete the unit conversion using dimensional analysis:
A • B/C
A: 3.33 mil air
B: 28.87 g air
C: 1 mol air

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calc...
Questions in other subjects:

Social Studies, 05.02.2020 02:52



History, 05.02.2020 02:52


Mathematics, 05.02.2020 02:52


Geography, 05.02.2020 02:52

Mathematics, 05.02.2020 02:52

Mathematics, 05.02.2020 02:52


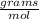 * 3.33 moles
* 3.33 moles



