
Chemistry, 07.10.2019 04:00, brookephillips1099
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen ions. a basic solution has a ph of 11.2. an acidic solution has a ph of 2.4. what is the approximate difference in the concentration of hydrogen ions between the two solutions?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1


Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen i...
Questions in other subjects:






English, 04.01.2021 19:50

History, 04.01.2021 19:50

Mathematics, 04.01.2021 19:50

Biology, 04.01.2021 19:50


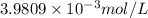
![pH=-\log[H^+]](/tpl/images/0295/9514/cf945.png)
![11.2=-\log[H^+]](/tpl/images/0295/9514/fa6ad.png)
![[H^+]=6.3095\times 10^{-12} mol/L](/tpl/images/0295/9514/45392.png)
![pH=-\log[H^+]'](/tpl/images/0295/9514/8468d.png)
![2.4=-\log[H^+]'](/tpl/images/0295/9514/13966.png)
![[H^+]'=0.39810 mol/L](/tpl/images/0295/9514/00ede.png)
![[H^+]'[H^+]](/tpl/images/0295/9514/81a76.png)
![[H^+]'-[H^+]=0.39810 mol/L-6.3095\times 10^{-12} mol/L=0.0039809 mol/L=3.9809\times 10^{-3} mol/L](/tpl/images/0295/9514/79396.png)




