
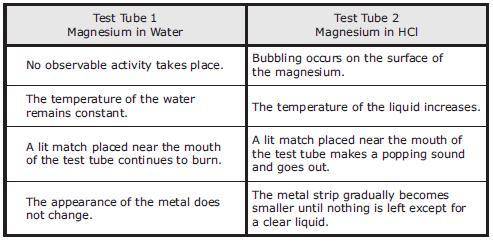
A student obtains two strips of magnesium, Mg, ribbon that are each 3 cm long. One strip of magnesium is placed in a test tube containing 5 mL of water, and the other strip is placed in a test tube containing 5 mL of hydrochloric acid, HCl. Both liquids are at room temperature. The student’s observations are recorded in the table.
Which statement is NOT supported by the student's observations?
Question 5 options:
A chemical reaction takes place between magnesium and hydrochloric acid.
A gas is released in Test Tube 2.
The substances in both test tubes are reactive only at high temperatures.
Energy is released in the reaction involving hydrochloric acid.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Do you know the correct answer?
A student obtains two strips of magnesium, Mg, ribbon that are each 3 cm long. One strip of magnesiu...
Questions in other subjects:

Biology, 22.04.2020 22:00


Mathematics, 22.04.2020 22:00



Mathematics, 22.04.2020 22:00









