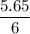Chemistry, 09.11.2020 01:50, Rocket3138
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the following reaction. Both reactant gases are initially at STP B2H6(g) + 6 Cl2(g) —> 3 BCl3(g) + 6HCl(g) Delta H = -1396 kJ

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the fo...
Questions in other subjects:

Biology, 09.11.2020 14:00


Mathematics, 09.11.2020 14:00

English, 09.11.2020 14:00

Business, 09.11.2020 14:00


English, 09.11.2020 14:00


English, 09.11.2020 14:00


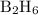 has been 58.637 kJ.
has been 58.637 kJ.