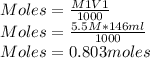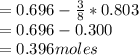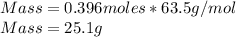Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is only one that occurs;
Cus) + HNO3(aq) → Cu(NO3)2(aq) + H2O() + NO()
Will the Cu react completely? What is the limiting reagent and what is the remaining compound
in mass?

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Q2. A 0.696 mol sample of Cu is added to 146 mL of 5.5 M HNO3. Assuming the following
reaction is o...
Questions in other subjects:


Chemistry, 09.12.2020 01:00


Medicine, 09.12.2020 01:00

English, 09.12.2020 01:00

History, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

English, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00