Chemistry, 08.11.2020 04:40, katiedurden02
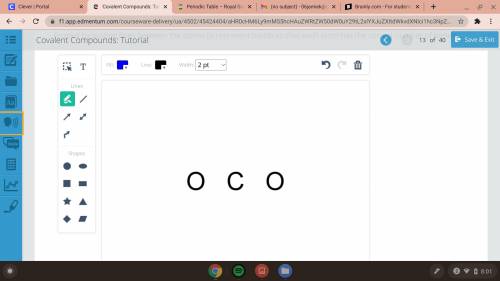
Carbon dioxide consists of a central carbon atom with an oxygen atom on each side. Draw a model of the molecule formed by putting lines between the atoms to represent bonds so that each atom has the correct number of bonds.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
Do you know the correct answer?
Carbon dioxide consists of a central carbon atom with an oxygen atom on each side. Draw a model of t...
Questions in other subjects:



Mathematics, 07.01.2021 18:00

Mathematics, 07.01.2021 18:00

Mathematics, 07.01.2021 18:00

Health, 07.01.2021 18:00

Mathematics, 07.01.2021 18:00


Health, 07.01.2021 18:00