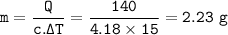Chemistry, 07.11.2020 20:50, HannahVance99
A sample of water is heated from 60.0 °C to 75.0°C by the addition of 140 j of
heat. What is the mass of the water?
work too pls !!!

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
Do you know the correct answer?
A sample of water is heated from 60.0 °C to 75.0°C by the addition of 140 j of
heat. What is the ma...
Questions in other subjects:




History, 20.12.2019 04:31





Mathematics, 20.12.2019 04:31

Biology, 20.12.2019 04:31