
Chemistry, 07.11.2020 01:00, katiekern5207
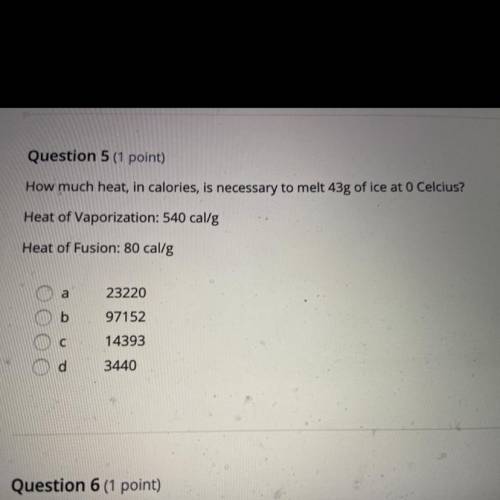
Question 5 (1 point)
How much heat, in calories, is necessary to melt 43g of ice at 0 Celcius?
Heat of Vaporization: 540 cal/g
Heat of Fusion: 80 cal/g
a
b
23220
97152
14393
с
d
3440

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1


Chemistry, 23.06.2019 10:30, 7thaohstudent
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
Do you know the correct answer?
Question 5 (1 point)
How much heat, in calories, is necessary to melt 43g of ice at 0 Celcius?
Questions in other subjects:


Chemistry, 30.11.2020 18:30


English, 30.11.2020 18:30



Mathematics, 30.11.2020 18:30

Physics, 30.11.2020 18:30







