
Chemistry, 06.11.2020 23:10, kokilavani
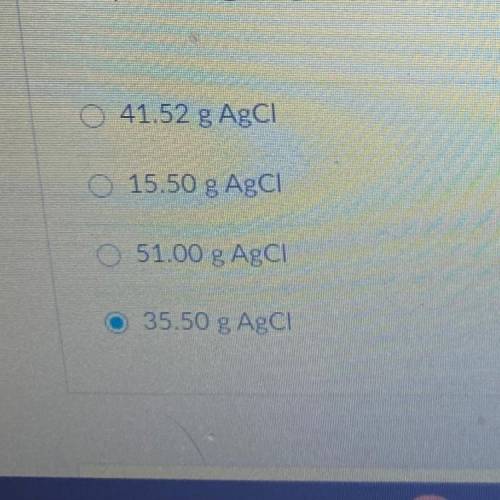
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is the percent yield of AgCl?
NH4Cl + AgNO3 --> AgCl + NH4NO3
69.61 % Yield AgCl
0 43.66 % Yield AgCl
85.50 % Yield AgCI
O 85.50 g AgCI

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
Do you know the correct answer?
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is...
Questions in other subjects:


Mathematics, 10.08.2021 23:00

Chemistry, 10.08.2021 23:00


Chemistry, 10.08.2021 23:00


Mathematics, 10.08.2021 23:00


Social Studies, 10.08.2021 23:00

Mathematics, 10.08.2021 23:00






