
Chemistry, 06.11.2020 20:00, thibeauxkristy
A sample of 87.6 g of carbon is reacted with 136 g of
fluorine gas to produce carbon tetrafluoride. Using
the balanced equation below, predict which is the
limiting reactant and the maximum amount in moles
of carbon tetrafluoride that can be produced.
C +2F2 → CF4
A. fluorine, 1.79 moles
B. carbon, 7.29 moles
C. fluorine, 6.72 moles
D. carbon, 4.63 moles

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
Do you know the correct answer?
A sample of 87.6 g of carbon is reacted with 136 g of
fluorine gas to produce carbon tetrafluoride....
Questions in other subjects:


World Languages, 21.12.2020 04:30


Mathematics, 21.12.2020 04:30

History, 21.12.2020 04:30

Mathematics, 21.12.2020 04:30

Mathematics, 21.12.2020 04:30



Mathematics, 21.12.2020 04:30


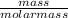
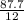 = 7.31moles
= 7.31moles 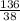 = 3.58moles
= 3.58moles 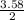 = 1.79moles of CF₄
= 1.79moles of CF₄



