Chemistry, 06.11.2020 16:40, ahnaodoido384
An excess of oxygen reacts with 451.4 g of lead, forming 374.7 g of lead(II) oxide. Calculate the percent yield of the reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, browndalton55
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Do you know the correct answer?
An excess of oxygen reacts with 451.4 g of lead, forming 374.7 g of lead(II) oxide. Calculate the pe...
Questions in other subjects:



History, 13.10.2020 05:01



Chemistry, 13.10.2020 05:01

English, 13.10.2020 05:01



Mathematics, 13.10.2020 05:01


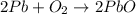
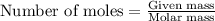
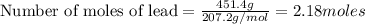
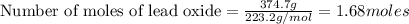
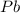 produces = 2 moles of
produces = 2 moles of 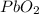
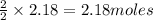 of
of