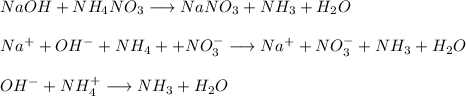Chemistry, 06.11.2020 16:20, toddbecca9
g Mark the statements as True or False. (1) When mixing, NaOH(aq) and NH4NO3 (aq) can not complete their reaction because there is no precipitate formation. (2) To quantify unknown soluble chloride, we could use either gravimetry or volumetric analysis based on AgCl(ppt) formation.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Do you know the correct answer?
g Mark the statements as True or False. (1) When mixing, NaOH(aq) and NH4NO3 (aq) can not complete t...
Questions in other subjects:



Mathematics, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00

English, 11.03.2021 14:00



Chemistry, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00