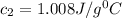Chemistry, 06.11.2020 16:30, halltristan657
You are performing an experiment where you place 6.7797 g of Al metal at 205.24 equationC into a coffee cup calorimeter that contains 87.4 g of water at 25.37 equationC. The final temperature of the water in the coffee cup is 28.67 equationC. Remember, the heat gained by the water is equal to the heat lost by the Al. equation What is the specific heat (C) of the Al in J/g°C? Cwater= 4.184 J/g°C. Do not include units. If you need to express your answer as an exponential number, use this template: 1445 should be typed as 1.445e+003

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
Do you know the correct answer?
You are performing an experiment where you place 6.7797 g of Al metal at 205.24 equationC into a cof...
Questions in other subjects:




Mathematics, 05.09.2020 01:01



Mathematics, 05.09.2020 01:01


Mathematics, 05.09.2020 01:01


 .
.
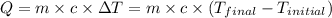
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/0874/2122/b0e58.png)
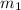 = mass of water = 87.4 g
= mass of water = 87.4 g 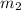 = mass of Al metal = 6.7797 g
= mass of Al metal = 6.7797 g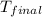 = final temperature =
= final temperature = 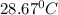
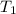 = temperature of water =
= temperature of water = 
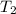 = temperature of Al metal =
= temperature of Al metal = 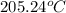
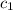 = specific heat of water =
= specific heat of water = 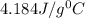
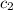 = specific heat of Al metal = ?
= specific heat of Al metal = ? ![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0874/2122/09236.png)
![87.4\times 4.184\times (28.67-25.37)^0C=-[6.7797\times c_2\times (28.67-205.24)]](/tpl/images/0874/2122/2d990.png)