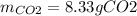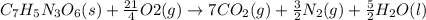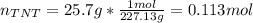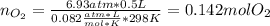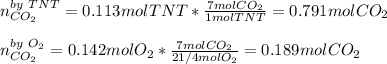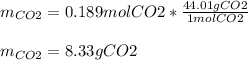Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced chemical equation:
C7H5N3O6(s)+214O2(g)→7CO2(g)+32N2(g )+52H2O(l)
If 25.7 g of TNT is combusted in a 0.500 L container filled with O2 at a pressure of 7.02 bar and a temperature of 298 K, calculate the maximum mass of CO2 that could be produced.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
Do you know the correct answer?
Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced ch...
Questions in other subjects:

English, 20.10.2021 02:10


Mathematics, 20.10.2021 02:10



History, 20.10.2021 02:10



Mathematics, 20.10.2021 02:10