
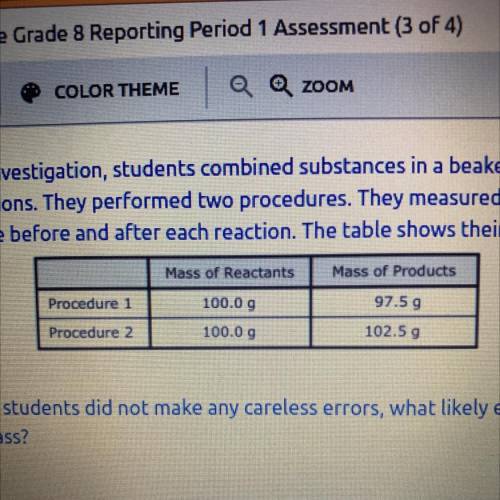
As part of an investigation, students combined substances in a beaker to observe
chemical reactions. They performed two procedures. They measured the mass of
each substance before and after each reaction. The table shows their observations.
Mass of Products
Procedure
97.59
Procedure 2
102.50
Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into
the air
Mass of Reactants
100.0 9
100.00
Procedure 1: One of the reactants was converted to thermal
energy,
Procedure 2: All the products were liquids.
e
Assuming the students did not make any careless errors, what likely explains these
changes in mass?
Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
e
air.
Procedure 1: One of the products was a gas that escaped into the
Procedure 2: A gas from the air reacted with one of the other
reactants and formed a precipitate.
CLEAR ALL

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1


Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3
Do you know the correct answer?
As part of an investigation, students combined substances in a beaker to observe
chemical reactions...
Questions in other subjects:

Mathematics, 22.04.2021 02:00

Business, 22.04.2021 02:00


Biology, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00



English, 22.04.2021 02:00






