
Chemistry, 05.11.2020 16:40, MendesMist
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is charged with 1.00 bar of CO. The gas is then pressurized with O2 to give a total pressure of 3.52 bar. The reactor is sealed, heated to 350 oC to drive the reaction to completion, and cooled back to 25.0 oC. Compute the final partial pressure of each gas (in bar).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
Do you know the correct answer?
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is ch...
Questions in other subjects:

Mathematics, 20.11.2019 05:31


Social Studies, 20.11.2019 05:31





Mathematics, 20.11.2019 05:31



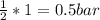 (required)
(required)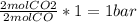 of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2
of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2



