Look at the image below. What is the ratio of the formula shown? *
0:0
1:1
1:2
2:...

Chemistry, 05.11.2020 14:00, kaylee0424
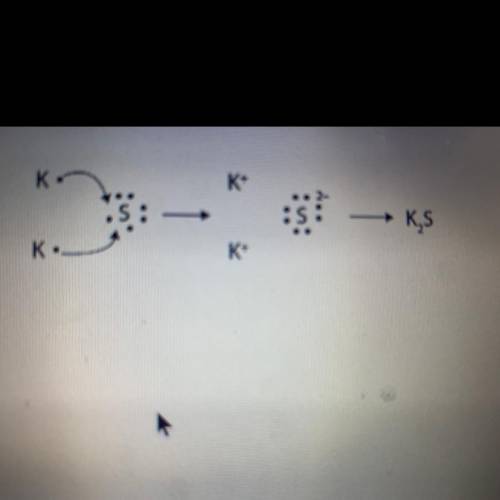
Look at the image below. What is the ratio of the formula shown? *
0:0
1:1
1:2
2:1
2:2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, shahedalahmad2017
What is common about these molecules? a. their atoms are held together by covalent bonds. b. they are all made up of the same two atoms. c. their atoms are held together by ionic bonds. d. they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Do you know the correct answer?
Questions in other subjects:

English, 26.09.2019 00:00

Physics, 26.09.2019 00:00



Computers and Technology, 26.09.2019 00:00

Computers and Technology, 26.09.2019 00:00


Chemistry, 26.09.2019 00:00

Mathematics, 26.09.2019 00:00

Mathematics, 26.09.2019 00:00






