
Chemistry, 04.11.2020 19:00, aredwolf2017
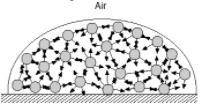
Surface tension is the inward pull that tends to minimize the surface area of a liquid. Water has high surface tension because of hydrogen bonding. In the diagram below, consider a molecule within the bulk of the liquid. This molecule experiences attractions to its neighboring molecules in all directions. These forces, shown by the arrows, average out to zero and there is no net force on the molecule. How is the situation different for a molecule at the surface? How does this give rise to surface tension?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
Do you know the correct answer?
Surface tension is the inward pull that tends to minimize the surface area of a liquid. Water has hi...
Questions in other subjects:

Medicine, 20.09.2020 20:01


Mathematics, 20.09.2020 20:01


Mathematics, 20.09.2020 20:01

Mathematics, 20.09.2020 20:01

Spanish, 20.09.2020 20:01

Chemistry, 20.09.2020 20:01

History, 20.09.2020 20:01

Social Studies, 20.09.2020 20:01






