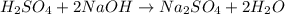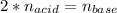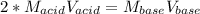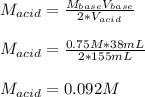Chemistry, 04.11.2020 19:00, joylsbarbour
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H2SO4). a. Write a balanced equation for the neutralizationof NaOH with H2SO4

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 13:30, bossboybaker
What happens to acetone molecules when you add heat to a beaker of liquid acetone?
Answers: 1
Do you know the correct answer?
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H...
Questions in other subjects:

World Languages, 14.04.2021 01:20






History, 14.04.2021 01:20


Mathematics, 14.04.2021 01:20

Chemistry, 14.04.2021 01:20