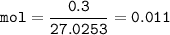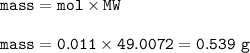Hydrogen Cyanide, HCN, is a poisonous gas. The
lethal dose is approximately
300mg HCN per kil...

Hydrogen Cyanide, HCN, is a poisonous gas. The
lethal dose is approximately
300mg HCN per kilogram of air when inhaled.
If the HCN is formed by the reaction of NaCN with an acid such as H2SO4,
what mass of NaCN gives the lethal dose in the room from the previous
question?
2NCN (s) + H2SO4 (aq) - Na2S04 (aq) + 2HCN (g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1


Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Mathematics, 09.02.2021 20:20


Mathematics, 09.02.2021 20:20


History, 09.02.2021 20:20



Mathematics, 09.02.2021 20:20

Mathematics, 09.02.2021 20:20