a. Label the x-axis as time.

Chemistry, 03.11.2020 21:40, carolyntowerskemp
Present Your Findings
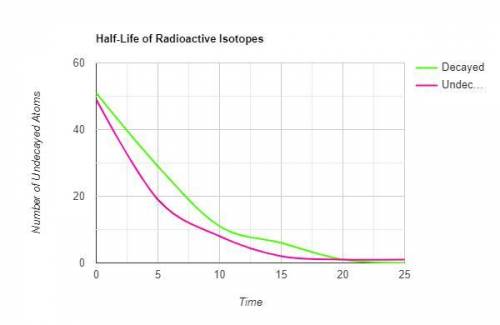
1. Make a graph of the data collected.
a. Label the x-axis as time.
b. Label the y-axis as the number of undecayed atoms.
c. Be sure to title the graph.
d. Turn the graph into your teacher.
2. Write a summary paragraph discussing this experiment and the results. Use the following questions to help guide the
content of your paragraph.
a. What was your hypothesis? According to your data, do you think your hypothesis was correct?
b. What is a half-life?
c. When does a radioactive sample emit the largest number of decay particles? Why is this information important?
(How can it be applied in our world today?)
d. Do you think the shape of the curve on your graph would change if you increased the half-life to 20 seconds?
What does this reveal about radioactive decay?
e. Do you think the shape of the curve on your graph would change if you started with a smaller sample, say 50
atoms (pennies)? What does this reveal about radioactive decay?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
Do you know the correct answer?
Present Your Findings
1. Make a graph of the data collected.
a. Label the x-axis as time.
a. Label the x-axis as time.
Questions in other subjects:

Geography, 05.07.2019 04:40

Mathematics, 05.07.2019 04:40

Arts, 05.07.2019 04:40

Arts, 05.07.2019 04:40

Computers and Technology, 05.07.2019 04:40




Chemistry, 05.07.2019 04:40