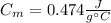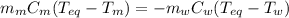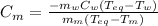Chemistry, 03.11.2020 17:20, Justadumbemo
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negligible heat capacity containing 80.0 mL water at 24.0 oC. The final temperature of the system was found to be 28.4 oC. Calculate the Specific heat of the metal if density of water is 1.00 g/ml.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Do you know the correct answer?
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negl...
Questions in other subjects:

Mathematics, 16.03.2020 02:44

Mathematics, 16.03.2020 02:44





Mathematics, 16.03.2020 02:44


Mathematics, 16.03.2020 02:44

History, 16.03.2020 02:44