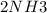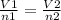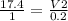Chemistry, 03.11.2020 17:00, diamoniquepowel
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L. The following reaction takes place: N2(g) + 3H2(g)2NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 07:30, apalacios3503
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
Do you know the correct answer?
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L...
Questions in other subjects:



Mathematics, 06.11.2019 10:31


Arts, 06.11.2019 10:31

Mathematics, 06.11.2019 10:31

Mathematics, 06.11.2019 10:31

Mathematics, 06.11.2019 10:31

Biology, 06.11.2019 10:31

Mathematics, 06.11.2019 10:31


 →
→