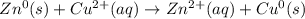Chemistry, 03.11.2020 16:50, bullockarwen
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent in the reaction of zinc with copper ions. Zn(s)+Cu2+(aq)⟶Zn2+(aq)+Cu(s) Which substance in the reaction gets reduced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
Do you know the correct answer?
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent...
Questions in other subjects:


Mathematics, 10.12.2020 03:10


Mathematics, 10.12.2020 03:10

Physics, 10.12.2020 03:10


Mathematics, 10.12.2020 03:10

Biology, 10.12.2020 03:10


Social Studies, 10.12.2020 03:10