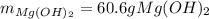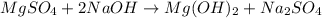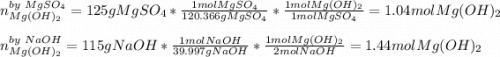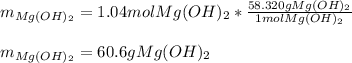Chemistry, 03.11.2020 16:40, babyleah2826
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hydroxide solution. If a solution containing 125 g of MgSO4 is added to a solution with 115 g of NaOH, what is the mass of milk of magnesia produced

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1


Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
Do you know the correct answer?
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hyd...
Questions in other subjects:



Mathematics, 07.06.2021 22:30



Biology, 07.06.2021 22:30

History, 07.06.2021 22:30


Mathematics, 07.06.2021 22:30

History, 07.06.2021 22:30