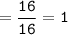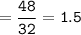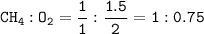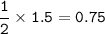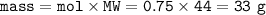Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
Do you know the correct answer?
How many grams of carbon dioxide are produced when 16.0
g of methane and 48.0 g of oxygen gas combu...
Questions in other subjects:


Arts, 04.12.2020 17:20








English, 04.12.2020 17:20