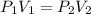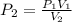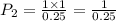Chemistry, 03.11.2020 05:00, ammarsico19
A 1.00 L sample of a gas at a pressure of 1.000atm is compressed to 0.25 L at a constanttemperature. What is the new pressure of the gas?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
A 1.00 L sample of a gas at a pressure of 1.000atm is compressed to 0.25 L at a constanttemperature....
Questions in other subjects:


Biology, 14.02.2020 18:21

Mathematics, 14.02.2020 18:21



Mathematics, 14.02.2020 18:21




English, 14.02.2020 18:22