
Chemistry, 02.11.2020 21:00, SoccerdudeDylan
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water so that the final volume of the solution is 2.50 L . Calculate the molarity of the MgCl2 solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2



Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
Do you know the correct answer?
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water...
Questions in other subjects:


History, 01.11.2019 06:31


Mathematics, 01.11.2019 06:31

Mathematics, 01.11.2019 06:31

Biology, 01.11.2019 06:31



Mathematics, 01.11.2019 06:31


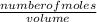
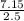 = 2.86mol/L
= 2.86mol/L



