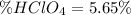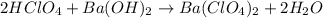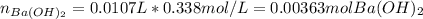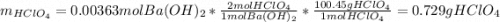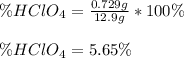Chemistry, 02.11.2020 17:20, sskibi1243
A 12.9 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. If 10.7 mL of 0.338 M barium hydroxide are required to neutralize the perchloric acid, what is the percent by mass of perchloric acid in the mixture

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3
Do you know the correct answer?
A 12.9 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. If...
Questions in other subjects:



Physics, 06.12.2019 15:31

Mathematics, 06.12.2019 15:31


Business, 06.12.2019 15:31