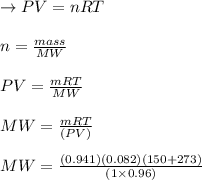A sample of an unknown compound is vaporized at . The gas produced has a volume of at a pressure of , and it weighs . Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1

Chemistry, 23.06.2019 13:20, dani9427
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3

Chemistry, 23.06.2019 14:20, ashleymunoz928
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
Do you know the correct answer?
A sample of an unknown compound is vaporized at . The gas produced has a volume of at a pressure of...
Questions in other subjects:

Mathematics, 12.10.2019 03:50

English, 12.10.2019 03:50



Mathematics, 12.10.2019 03:50

Mathematics, 12.10.2019 03:50





 "
"