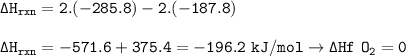Chemistry, 02.11.2020 14:00, steve12335
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combustion of a gram of hydrocar-
bon is between 40 and 50 kJ.
Practice Exercise 1
Calculate the enthalpy change for the reaction
2 H2O2(l)-→ 2 H2O(l) + O2(g)
using enthalpies of formation:
Change in enthalpy(f)H2O2(l)= -187.8 kJ/mol
Change in enthalpy (f) [H2O(l)= -285.8 kJ/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
Do you know the correct answer?
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combusti...
Questions in other subjects:

Mathematics, 04.03.2021 03:10

Mathematics, 04.03.2021 03:10


Mathematics, 04.03.2021 03:10



Mathematics, 04.03.2021 03:10

Mathematics, 04.03.2021 03:10