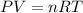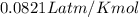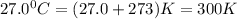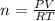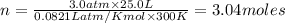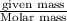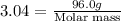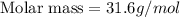Chemistry, 02.11.2020 08:10, GreenHerbz206
The gaseous product of a reaction is collected in a 25.0L container at 27.0 C. The pressure in the container is 3.0atm and the gas has a mass of 96.0g. What is the molar mass of the gas?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, 4tazaouiamine1r
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
Do you know the correct answer?
The gaseous product of a reaction is collected in a 25.0L container at 27.0 C. The pressure in the c...
Questions in other subjects:


Mathematics, 10.02.2021 01:20

Mathematics, 10.02.2021 01:20

Mathematics, 10.02.2021 01:20

History, 10.02.2021 01:20




Biology, 10.02.2021 01:20