Chemistry, 02.11.2020 07:00, momneedshelphmwk
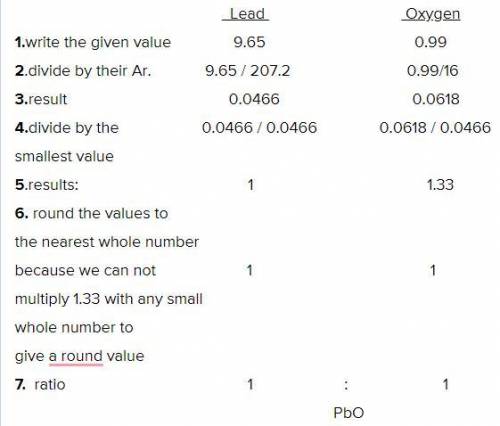
10.64 g sample of a lead compound is analyzed and found to be made up of 9.65 g of lead and 0.99 g of oxygen. Determine the empirical formula for this compound.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
Do you know the correct answer?
10.64 g sample of a lead compound is analyzed and found to be made up of 9.65 g of lead and 0.99 g o...
Questions in other subjects:


Mathematics, 21.09.2021 23:20



Spanish, 21.09.2021 23:20


Medicine, 21.09.2021 23:20


Mathematics, 21.09.2021 23:20

Biology, 21.09.2021 23:20