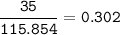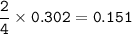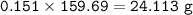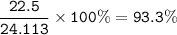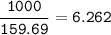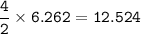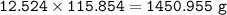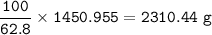Chemistry, 02.11.2020 06:30, Svetakotok
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What is the percentage yield of the reaction?
b) What mass of FeCO3 with a purity of 62.8% is needed to make 1.00 kg of Fe2O3?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Do you know the correct answer?
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What...
Questions in other subjects:

History, 19.10.2020 09:01





English, 19.10.2020 09:01


Social Studies, 19.10.2020 09:01