

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
Do you know the correct answer?
Determine the mass (in grams) of C2H6O necessary to produce 12.0 g CO2 in the following reaction:
C...
Questions in other subjects:


Computers and Technology, 21.08.2019 16:00

History, 21.08.2019 16:00

History, 21.08.2019 16:00


Mathematics, 21.08.2019 16:00

Social Studies, 21.08.2019 16:00

History, 21.08.2019 16:00

Mathematics, 21.08.2019 16:00

Mathematics, 21.08.2019 16:00


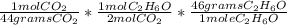 =6.3 grams of C2H6O
=6.3 grams of C2H6O



