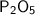How many atoms of each element are in the chemical formula P2O5?
2 phosphorus and 5 oxygen
5...

Chemistry, 01.11.2020 05:00, morganmattal
How many atoms of each element are in the chemical formula P2O5?
2 phosphorus and 5 oxygen
5 phosphorus and 2 oxygen
1 phosphorus and 5 oxygen
2 phosphorus and 1 oxygen

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1


Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
Do you know the correct answer?
Questions in other subjects:





Mathematics, 16.09.2021 20:00





Biology, 16.09.2021 20:10