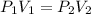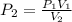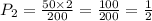Chemistry, 30.10.2020 20:20, Jimenezmiranda
A gas has a volume of 50mL at a pressure of 2 atm. Increase the volume to 200mL, and the temperature remains constant. What is the new pressure?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
Do you know the correct answer?
A gas has a volume of 50mL at a pressure of 2 atm. Increase the volume to 200mL, and the temperature...
Questions in other subjects:

Mathematics, 28.08.2021 01:00




Mathematics, 28.08.2021 01:00


Biology, 28.08.2021 01:00

Mathematics, 28.08.2021 01:00

Mathematics, 28.08.2021 01:00

Social Studies, 28.08.2021 01:00