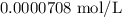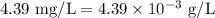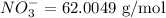Chemistry, 30.10.2020 16:50, deepspy599otchpd
A sample has a nitrate concentration of 4.39 mg/L (4.39 ppm nitrate). Calculate its molar concentration (in moles/L). (This primarily involves unit conversions).

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
Do you know the correct answer?
A sample has a nitrate concentration of 4.39 mg/L (4.39 ppm nitrate). Calculate its molar concentrat...
Questions in other subjects:



English, 24.10.2019 06:43

English, 24.10.2019 06:43


Spanish, 24.10.2019 06:43

History, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43


Biology, 24.10.2019 06:43