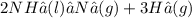Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
El amoniaco se descompone en nitrógeno e hidrógeno, ambos en estado gaseoso. N=14 H=1 Calcula los mo...
Questions in other subjects:

Arts, 09.12.2021 21:10



Arts, 09.12.2021 21:10

History, 09.12.2021 21:10





Physics, 09.12.2021 21:10