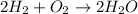Chemistry, 31.01.2020 07:50, sbhunsaker9025
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total mass of the reactants in a chemical reaction will never be equal to the total mass of the products. b. the total mass of the reactants in a chemical reaction will be significantly more than the total mass of the products. c. the total mass of the reactants in a chemical reaction is conserved and will be equal to the total original mass of the products. d. the total mass of the reactants in a chemical reaction will be significantly less than the total mass of the products.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total...
Questions in other subjects:

Mathematics, 09.03.2021 04:40


Social Studies, 09.03.2021 04:40


Mathematics, 09.03.2021 04:40

History, 09.03.2021 04:40



History, 09.03.2021 04:40