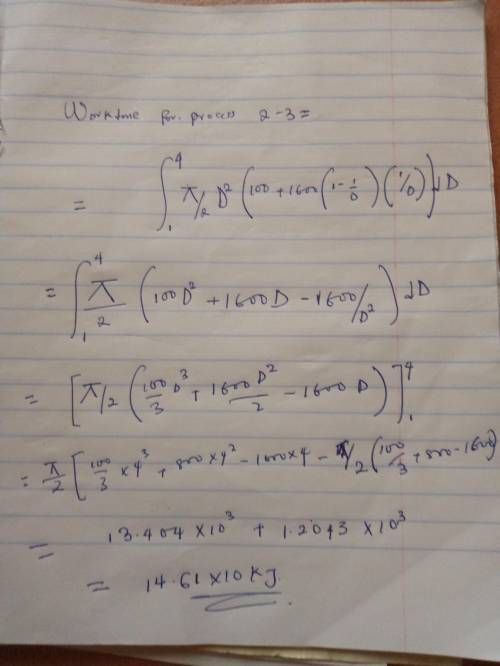Chemistry, 28.10.2020 16:40, adambbogard1589
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium gas at 1 MPa at ambient temperature of 20 degrees C. The valve is open and the balloon is inflated at constant pressure of 100 kPa (atmospheric pressure) until it becomes spherical at D1 = 1m. If the balloon is larger than this, the balloon material is stretched giving a pressure inside as:
P = Po + C(1-(D1/D))(D1/D)
The balloon is slowly inflated to a final diameter of 4m, at which point the pressure inside is 400 kPa. The temperature remains constant at 20 degrees C. Determine the work done during the overall process.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1

Do you know the correct answer?
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium g...
Questions in other subjects:

Biology, 23.07.2019 02:30




Chemistry, 23.07.2019 02:30