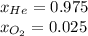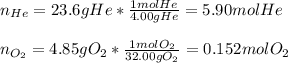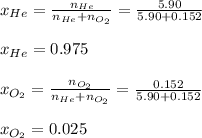Chemistry, 28.10.2020 16:30, dontcareanyonemo
A 13.0-L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 23.6 g of He and 4.85 g of O2 at 298 K. Calculate the mole fraction of each component in the mixture.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1


Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
Do you know the correct answer?
A 13.0-L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 23.6 g of He and 4.8...
Questions in other subjects:

Health, 11.03.2021 02:40

Mathematics, 11.03.2021 02:40



Mathematics, 11.03.2021 02:40

English, 11.03.2021 02:40