Chemistry, 27.10.2020 21:30, mbonham481
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temperature be (°C) with a pressure of 1.80 atm and constant volume?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
Do you know the correct answer?
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temper...
Questions in other subjects:

Physics, 27.09.2021 01:50

Biology, 27.09.2021 01:50

Mathematics, 27.09.2021 01:50


Computers and Technology, 27.09.2021 01:50

Mathematics, 27.09.2021 01:50

Computers and Technology, 27.09.2021 01:50

Mathematics, 27.09.2021 01:50



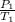 =
= 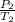
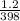 =
=