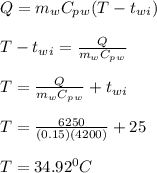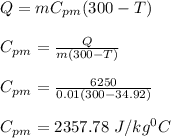Chemistry, 27.10.2020 19:10, Baby010391
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature water that was initially 25.0 °C. If 6.25 kJ of heat was transferred, what was the final temperature of the water?What was the specific heat of the metal?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2


Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
Do you know the correct answer?
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature...
Questions in other subjects:

Geography, 22.04.2021 23:00






Mathematics, 22.04.2021 23:00

Mathematics, 22.04.2021 23:00



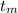 = 300 °C
= 300 °C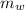 = 150 g = 0.15 kg
= 150 g = 0.15 kg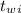 = 25.0 °C
= 25.0 °C