
Chemistry, 26.10.2020 18:00, EstevanOtero7361
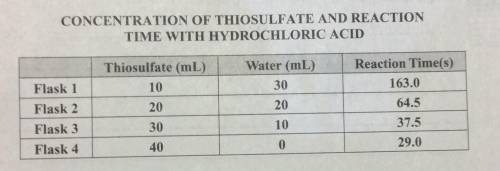
A researcher collected the data shown to see how the concentration of thiosulfate in water affects the reaction time with hydrochloric acid. Which conclusion can the researcher draw?
A
As concentration increases, reaction time decreases.
B
There is no obvious mathematical relationship.
C
As concentration decreases, reaction time decreases.
D
As concentration increases, reaction time increases.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Do you know the correct answer?
A researcher collected the data shown to see how the concentration of thiosulfate in water affects t...
Questions in other subjects:


English, 23.05.2020 06:57





History, 23.05.2020 06:57








