
Chemistry, 26.10.2020 17:00, datboyjulio21
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What is the limiting reagent in the reaction and what is the percent theoretical yield?

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What i...
Questions in other subjects:

Mathematics, 03.03.2021 04:40


Mathematics, 03.03.2021 04:40

Mathematics, 03.03.2021 04:40

Mathematics, 03.03.2021 04:40




Spanish, 03.03.2021 04:40

English, 03.03.2021 04:40


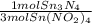 = 0.112 mol Sn₃N₄
= 0.112 mol Sn₃N₄



