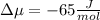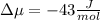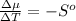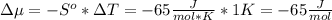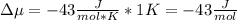Chemistry, 26.10.2020 16:40, corey36dylon
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the same temperature is 43 J K−1 mol−1. Calculate the change in chemical potential of liquid water and of ice when the temperature is increased by 1 K from the normal melting point. Giving your reasons, explain which phase is thermodynamically the more stable at the new temperature.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
Do you know the correct answer?
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the sam...
Questions in other subjects:


English, 04.02.2020 09:45

History, 04.02.2020 09:46



Mathematics, 04.02.2020 09:46




Mathematics, 04.02.2020 09:46