
Chemistry, 23.10.2020 17:20, alexwlodko
The specific heat capacity of water is 4.18 J/(g•°C). When 200 grams of water cools from 50.°C to 25°C, the total amount of heat energy released by the water is

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
The specific heat capacity of water is 4.18 J/(g•°C). When 200 grams of water cools from 50.°C to 25...
Questions in other subjects:











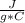 m= 200 gΔT= Tfinal - Tinitial= 50°C - 25°C= 25°C
m= 200 gΔT= Tfinal - Tinitial= 50°C - 25°C= 25°C




