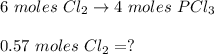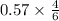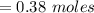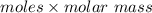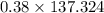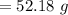Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1
Do you know the correct answer?
A 12.39 g sample of phosphorus reacts with 40.75 g of chlorine to form only phosphorus trichloride (...
Questions in other subjects:




Mathematics, 13.04.2020 20:18


Mathematics, 13.04.2020 20:18



Mathematics, 13.04.2020 20:18

History, 13.04.2020 20:18



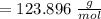
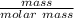
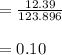
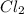 molar mass
molar mass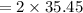
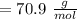
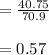
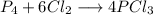
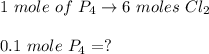
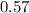 ,
, 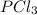 Theoretical performance
Theoretical performance