A silver ring reacts with compounds containing sulfur in the air to form silver sulfide,
a bla...

Chemistry, 22.10.2020 21:01, ansbert289
A silver ring reacts with compounds containing sulfur in the air to form silver sulfide,
a black substance that makes up the tarnish on the surface of silver objects. To
remove the tarnish from the ring, students placed it in a pan lined with aluminum foil
and added hot water. Baking soda was added to the hot water and stirred. Students
made observations about the process.
Which observation of this process provides evidence of a chemical reaction?
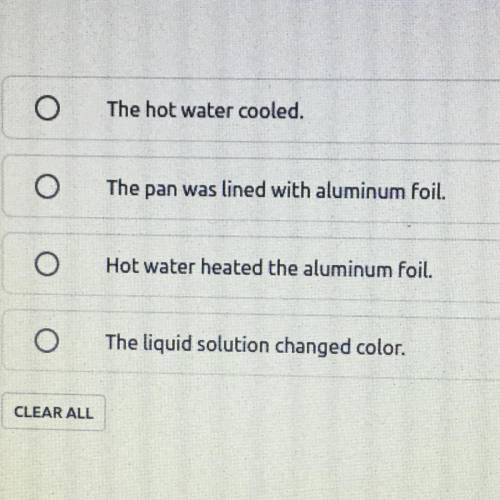

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
Do you know the correct answer?
Questions in other subjects:



Mathematics, 03.08.2019 07:40


Chemistry, 03.08.2019 07:40





History, 03.08.2019 07:40






